
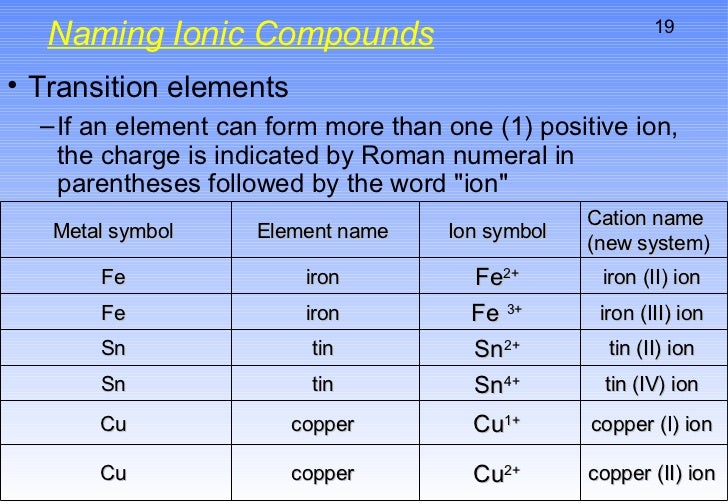
Nonmetal Atoms Gain Electrons The outermost. Explanation: And thus metals tend to form positive ions. The equation can also be written using the charge of each ion, expressed in coulombs (C), incorporated in the constant. Only a small amount of energy is needed to take electrons from metal atoms.
ARE METALS POSITIVE OR NEGATIVE IONS FULL
This value of k includes the charge of a single electron (1.6022 × 10 −19 C) for each ion. When it comes to metal ions, there is a bit of debate over whether they are positive or negative. Metal atoms lose electrons from their outer shell when they form ions: the ions are positive, because they have more protons than electrons the ions formed have full outer shells the. The proportionality constant k is equal to 2.31 × 10 −28 J Generating Ionic Bonds Ionic bonds form when metals and non-metals chemically react. Where each ion’s charge is represented by the symbol Q. The metals form positively-charged ions and the non-metals form negatively-charged ions. This action repeats itself and the bell rings.\] The electric current is then restored in the circuit and again the striker hits the gong by the above process. Oxygen (from the OH - in water) is produced unless the negative ion is one of. At this point, the electromagnet loses its magnetism and the iron strip moves back and comes in contact with the contact screw. The only metals less reactive are copper, silver, gold and platinum. As soon as the striker hits the gong, the screw loses its contact with the iron strip and therefore, current stops in the circuit. This magnetised copper wire (or the electromagnet) attracts the iron strip towards it, letting the striker hit the gong and thus sound is produced. When the switch is 'ON' and the screw is in contact with the iron strip, then electric current flows through the copper wire which gets magnetised because of electromagnetism. Striker in touch with contact screw through an iron strip.Electromagnet: A copper wire is wound around an iron piece which acts a magnet when current flows through it.Page No 27:įollowing are the components of electric bell: The life of dry cell is longer than cells using liquid electrolyte.

Usefulness of dry cell: They are handy and portable. Because of this, electric charge is produced on the two terminals of the cell and electric current flows in the circuit. Working of dry cell: Chemical reactions take place between the electrolyte, zinc container and graphide rod. It acts as the positive terminal of the cell.
It is surrounded by paste of Manganese dioxide (MnO 2). Question Explain why, in a test for halide ions, dilute hydrochloric acid cannot be used to acidify the. Metal rod: There is a graphite rod at the centre of the cell. This removes them, so stopping them giving an incorrect positive result for chloride ions.

It is the charge carrier of electricity as it contains negatively charged and positively charged ions. Electrolyte is a wet pulp of Zinc chloride (ZnCl 2) and Ammonium chloride (NH 4Cl). It acts as a negative terminal of the cell.Įlectrolyte: Inside the Zinc metal, there is the electrolyte filled between two layers. Outer metal covering: The metal covering is made up of zinc metal and is whitish in appearance. The dry cell consists of following components:


 0 kommentar(er)
0 kommentar(er)
